WHITE PAPER
Assess Stability and Material Compatibility of Biopharmaceuticals
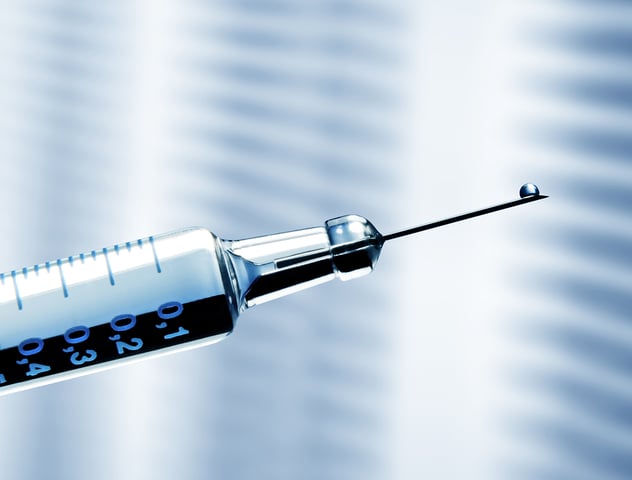
Over their lifespan, from manufacturing to storage and administration, biopharmaceutical drugs will interact with many different surfaces, where every interface encounter, presents a risk of adsorption, loss of concentration, and/or formation of proteinaceous particles. Late discovery of incompatibilities may not only jeopardize product development timelines, but it could also pose large financial costs. QSense QCM-D technology can be used to assess the adsorption of biologics and excipients to gain insight into when and why incompatibilities could occur and to identify ways to mitigate them.
In this white paper, we show examples where QSense was used to assess mAb adsorption on container closure materials relevant for biologics production with emphasis on pre-filled syringes, as well as the effect of added surfactant.

In this white paper
- glass (syringe barrel)
- plastics (cap)
- silicon oil (plunger lubrication)
- metal (needle)
